Live Longer with Alternate Fasting
Intermittent fasting may help increasing life span
First published: 11.Jan.2019
Several scientific studies involving different types of living beings (mice, yeasts, nematode worms, and flies) show that intermittent fasting can extend lifespan.
Whether this applies to human beings is still under discussion, but the prospect of eating less and living longer is indeed interesting.
This article focuses on scientific research investigating animal how animal lifespan increases when they follow a calorie-restricted diet.
It also looks into the probable mechanisms by which diet (and exercise) can influence lifespan and its implications for human longevity.

Fasting and a longer life for rats
It was Clive Main McCay, a professor at Cornell University, who published the first report linking the life span of rats with caloric restriction in 1934 (1). He worked with animal husbandry and had noticed how a reduced-calorie diet contributed to lengthening animal life spans.
Anton J. Carlson published a study in 1946 (2) that showed that "intermittent fasting" extended the lives of male rats by about 20% and those of females by 15%.
He also pointed out that "the development of mammary tumors was retarded in proportion to the amount of fasting," suggesting that alternate fasting had a cancer-protective effect.
Since then, the effect of fasting on extending life span has been verified in a wide variety of living beings: from yeast to worms; and from rats to flies and monkeys.
But what about humans?
Fasting and Human Health
Restricting caloric intake in human beings appears to increase the body's resistance to oxidative stress, and this may be the reason why dietary restriction has beneficial effects on aging.
Oxidative Stress
Your body produces reactive compounds called "Free Radicals" (also known as "reactive oxygen species", or ROS), which actively react with other molecules and disrupt your body's metabolism and cellular structures such as protein, fat, membranes, and even your DNA, by "oxidizing" them.
They create more free radicals during oxidation in a vicious circle known as "oxidative stress".
Smoking, alcohol, stress, contaminants, and drugs are factors that produce ROS. Even exercise creates them, but the problem arises when ROS are produced in quantities that overwhelm your body's natural antioxidant mechanisms which are supposed to neutralize free radicals.
Aging and Free Radicals
Some aging theories propose that free radicals degrade the mitochondria, tiny organelles inside your cells, that produce the energy required by your body.
This gradual decay due to oxidation leads to aging and death.
Fasting is beneficial for your health
Observational studies (those that record data from real-life situations without intervention by the investigator - meaning that the actual cause or mechanism is unknown) show that people who routinely fast for religious reasons, such as Greek Orthodox Christians, Muslims during Ramadan, and Christians during the Daniel Fast, had healthier cardiovascular systems and health risk parameters than people on a regular diet:
For instance, Greek Orthodox Christians fast for about 190 days each year eating a variant of a vegetarian diet. The reported outcome for them was that fasting led to "the lowering of body mass, total cholesterol, [bad] LDL-C[holesterol], and the LDL-C/HDL-C ratio." (3)
Those following a Christian Daniel Fast (commonly lasting 21 days) showed improved insulin sensitivity, lower blood lipids (fats), and blood pressure, as well as lower oxidative stress.
Reduced inflammation, cholesterol, blood pressure, insulin resistance, obesity, and oxidative stress can all contribute towards longevity because they improve your health.
But can you expect dietary restriction to act in the same way in you, as it does in mice?
Unfortunately, humans are not mice
There are metabolic differences between human beings and rodents (4). For instance, a lower caloric intake in mice reduces the concentration of IGF-1 by around 35%, and this may have a positive impact on their longevity.
IGF-1 (insulin-like growth factor-1) has growth-promoting effects on nearly all body cells from muscle, to the bone, to organs, and skin.
A lower presence of IGF-1 "is linked to improved longevity [and in humans,] individuals with exceptional longevity are enriched with functional IGF-1R mutations, while low IGF-1 levels predict better survival in female nonagenarians" (5), meaning that less IGF-1 promotes longer life.
So mice on a low-calorie diet have 35% less IGF-1 in their bloodstream and live longer, but reduced caloric diets in healthy humans don't have any effect on IGF-1 concentrations "unless protein intake is also reduced." So maybe a diet with less protein can have a life-extending effect in humans (4).
Summarizing Fasting in Humans
Fasting in humans is good for overall health and, coupled to a lower protein intake -to reduce IGF-1 concentrations, could help improve longevity.
The problem is that most fasting studies in humans have concentrated on the weight loss aspect and not on its life-extending effects.
We will look into intermittent fasting and its weight loss effects in another article.
In the next section, we will try to understand the link between fasting and life span.
Effects of fasting on aging
Better immune systems
Recent research (Neff, Xie, 2017) (6) studied the effect of every-other-day feeding (EOD) in mice and found that it extended the average lifespan between 12 and 27%.
This study also looked into how these mice aged; that is, they investigated how alternate fasting inhibited the negative side-effects of aging. This is what they found:
- Alternate fasting delayed the appearance of neoplastic disease (that means that both benign and malignant tumor formation was delayed, appearing at an older age).
- There was only a limited delay of aging, meaning that aging continues roughly at the same pace.
- Bone loss, joint stiffness, osteoporosis, age-dependent hearing loss, cataract formation were the same in EOD mice, and non-fasting mice.
- On the positive side, EOD reduced heart weight and size and had a positive impact on the immune system (which degrades with aging), thus preventing infections, autoimmune diseases, and maybe even cancer.
Of course, dieting (that is, eating a diet with fewer calories, say 20% less daily) also extends lifespan but, an intermittent fasting diet is not so reduced in calories (in this mice experiment they only ate 7.5% fewer calories over their whole lifespan). This makes alternate fasting more bearable than a low-calorie diet for the long term.
The EOD mice also had a bodyweight that was 17.5% lower than mice on a normal diet.
These mice lived 102 days longer (total lifespan 908 days) than normally fed mice, who only lived 806 days.
Take-home point
Intermittent fasting mice show age-decay at the same rate as normally fed ones but have better immune systems and later onset of tumors (benign and malignant)
They are slimmer and live up to 27% longer because cancer kills them later.
Biological mechanisms that explain the effects of fasting on lifespan
What is the mechanism linking fasting to a longer life?
Scientists are trying to identify how a calorie-restricted lifestyle influences longevity. In other words, what are the biochemical or metabolic mechanisms involved?
A study published by Harvard researchers (Weir et al. 2017) (7) studied a nematode worm, Caenorhabditis elegans, a very convenient subject because it only lives for two weeks.
They found that the worms' mitochondria -minute organelles located in the cells' nucleus that produce the cell's energy- are linked to lifespan.
Mitochondria form networks within the nucleus, and can exist in a linked (fused) state or a fragmented (fissioned) state depending on the cell's energy requirements.
Fused mitochondria are more youthful
Fragmented or fissioned mitochondria appear when they suffer oxidative stress and self-destruct. Also, when cells divide, splitting-off daughter mitochondria for the newly formed cell.
Mitochondrial fusion is required for preventing cell death in mammals and for maintaining their respiratory capacity and their ability to produce energy (membrane potential). So a fused state is far more beneficial than a fissioned one.
Fused mitochondria are elongated.
As the cells age, the mitochondria become less "flexible" and tend to remain in one of these two states.
However, through dietary restriction, Weir's team maintained the mitochondria in the "fused" state which is a more "youthful" one.
AMPK, fasting, mitochondria, and aging
Aging also reduces the cell's capacity to activate an enzyme called "AMP-activated protein kinase" or AMPK. This enzyme regulates the uptake of glucose and fatty acid by the mitochondria (that "burns" them to generate energy for the cell).
"Many studies with lower organisms have revealed that increased AMPK activity can extend the lifespan" (8), and in mammals, it maintains cellular health by upkeeping the "cellular housekeeping" processes in order.
It also "improves cellular stress resistance [and] suppresses inflammatory responses" (8).
AMPK's loss of sensitivity with age leads to an increase in oxidative stress and triggers low-grade inflammation that impacts negatively on the aging process.
Fasting and dieting (caloric restriction) stimulate the activity of AMPK while "nutritional overload seems to impair AMPK activity and concurrently induce insulin resistance in many tissues thus promoting the appearance of the components of the metabolic syndrome i.e. obesity, diabetes and cardiovascular diseases." (8)
So there is a link between eating less and enhanced AMPK sensitivity that in turn combats inflammation and oxidative stress, and promotes longevity.
Overeating, on the other hand, inhibits the activity of AMPK, leading to metabolic syndrome, and a shorter lifespan.
Weir's team (7) genetically manipulated the activation of AMPK and found that it kept the mitochondrial networks in a healthy "fused" state. And that these more youthful mitochondrial networks regulated the metabolism of fat by communicating with other cellular organelles called peroxisomes. The scientists reported that "longevity pathways require coordination between mitochondria and peroxisomes."
These peroxisomes also contributed to "increase[d] lifespan via fatty acid oxidation."
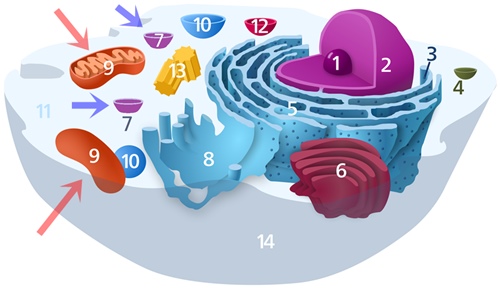
Parts of a typical animal cell
- Nucleolus
- Nucleus
- Ribosome
- Vesicle
- Rough endoplasmic reticulum
- Golgi apparatus
- Peroxisome (blue arrows)
- Smooth endoplasmic reticulum
- Mitochondrion (red arrows)
- Vacuole
- Cytosol
- Lysosome
- Centrosome
What are Peroxisomes?
Peroxisomes are organelles that live in the cellular cytoplasm and their main function is to break down fatty acids (fats and oils) which are used either as fuel by the mitochondria -or to repair cellular membranes.
This process produces a hydrogen ion, which reacts with oxygen to produce hydrogen peroxide (like the one you have in your bathroom cabinet) which is a highly reactive ROS that the peroxisome quickly converts into harmless water.
So the role of peroxisomes is critical in fuelling mitochondria and keeping some oxidants under control.
Fransen, Lismont, and Walton (2017) (9) reported that peroxisomes also served as signaling platforms that acted upon processes such as immunity and inflammation, and that alterations in these signaling functions could lead to many diseases and also to "more common age-related disorders such as diabetes, neurodegenerative disease, and cancer."
Healthy individuals are the result of the concerted action of both organelles, peroxisomes, and mitochondria, so a coordinated action such as that mentioned by Weir is fundamental.
Fransen's team was cautious, and acknowledged that for now "little is known about how disturbances in the bidirectional communication ... between peroxisomes and mitochondria influence age-related disease development."
What is known is that exercise plays an important role in mitochondrial fusion:
Exercise and mitochondria
Mice that exercise by running, display an increase in mitochondrial fusion, and C. elegans nematodes that are made to swim as an exercise, live longer and also display an increase in the quantity of "elongated mitochondria" (that are fused together) (9).
So, elongated mitochondria may mean a longer lifespan
Fransen et al. suggested that "increased mitochondrial elongation positively contributes to exercise-induced longevity."
Fused, or elongated mitochondria are more efficient, furthermore, they produce more of the energy-rich molecule adenosine triphosphate (ATP) which is necessary for the survival of elderly long-lived nematodes.
Below we will see how ATP affects longevity.
Lack of energy is a probable cause of aging
Chaudhari and Kipreos (2017) (10) proposed the "Energy Maintenance Theory of Aging", which states that the survival of older animals requires adequate energy levels.
They published a paper that ratified that increased mitochondrial fusion promoted longevity, at least in "C. elegans" nematodes.
One theory about aging suggests that cellular damage due to the free radicals produced by aging mitochondria causes shorter lifespans.
But, according to Chaudhari and Kipreos, old mitochondria don't produce enough ATP and the lower amount of energy does not allow the cell to repair itself, leading to cellular aging and eventually death.
This theory brings together many of the life-extending factors we have mentioned in this article:
- Restricted calories cause mitochondrial fusion: "nutrient limitation increases mitochondrial fusion" (10).
- Elongated mitochondria are linked to longevity: the researchers "observed an increase in elongated mitochondria in diverse longevity pathways, including: ...caloric restriction; .... AMPK overexpression ... and exercise... These observations suggest that increased levels of elongated mitochondria are broadly associated with longevity."
- "Elongated morphology is associated with increased efficiency of ATP production and reduced generation of reactive oxygen species (ROS), while fragmented morphology is linked to reduced ATP production and mitochondrial uncoupling."
So exercise, eating less, abundant AMPK, and good genes all contribute to elongated mitochondria and this, in turn, leads to an efficient output of ATP (energy) and less free radicals (ROS), which contribute to a longer life.
How exercise and fasting act upon mitochondria is still a mystery, which further investigation should help to clarify.
Closing comments
Clinical and animal studies have shown that modulating diet and meal frequency, as well as the application of fasting patterns, e.g. intermittent fasting, periodic fasting, or long-term fasting, are part of a new lifestyle approach leading to increased life and health span enhanced intrinsic defences against oxidative and metabolic stresses, improved cognition, as well as a decrease in cardiovascular risk in both obese and non-obese subjects. Wilhelmi de Toledo F, Grundler F, Sirtori CR, Ruscica M., (2020) (11)
Research involving rodents and worms have shown that fasting and exercising extend their lifespans.
The mechanism involves mitochondria, tiny organelles located in every cell, which produce the energy that the cells and the body require to be able to live.
Youthful, elongated, or fused mitochondria are more efficient in producing energy-rich ATP molecules, they also produce less free radicals (ROS) -reducing oxidative stress- and have smooth coordination with peroxysomes, organelles that supply the mitochondria with fuel and also neutralize ROS.
Fasting also stimulates an enzyme called AMPK which helps the mitochondria absorb their fuel and also prevents inflammation and helps control oxidative stress.
Lower oxidative stress and inflammation mean better immunity, lower risk of disease (diabetes, metabolic syndrome, cardiovascular disease), and better health parameters (lower cholesterol, blood lipids, blood pressure, and better insulin response).
So several cellular mechanisms are involved in longevity and exercise coupled with a low calorie, or an alternate fasting diet helps to keep them running smoothly.
Take-home point
Intermittent fasting and exercise may help you to live longer.
They will surely help you to lose weight and improve your health.
References and Further Reading
(1) M. McCay, C & F. Crowell, Mary. (1934). Prolonging the Life Span. The Scientific Monthly. 39. 405-414
(2) Anton J. Carlson & Frederick Hoelzel, (1946).Apparent Prolongation of the Life Span of Rats by Intermittent Fasting: One Figure. The Journal of Nutrition, Vol 31:3, 1 March 1946, 363-375, https://doi.org/10.1093/jn/31.3.363
(3) John F Trepanowski & Richard J Bloome, (2010). The impact of religious fasting on human health. 2010 Nov 22. doi: 10.1186/1475-2891-9-57
(4) Fontana L, Partridge L, Longo VD, (2010). Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321-6
(5) Kai Mao et al., (2018). Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nature Communications vol 9, Article number: 2394 19 June 2018
(6) Kan Xie, Frauke Neff, et al., (2017)Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nature Communications vol 8, Article number: 155 (2017)
(7) Heather J. Weir, et al., (2017). Dietary Restriction and AMPK Increase Lifespan via Mitochondrial Network and Peroxisome Remodeling. Cell Metabolism Vol 26:6, 884-896.E5, Dec 05, 2017 DOI:https://doi.org/10.1016/j.cmet.2017.09.024
(8) Antero Salminena, Kai Kaarniranta, (2010). AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Research Reviews, Vol 11:2, April 2012, 230-241 https://doi.org/10.1016/j.arr.2011.12.005
(9) Fransen M, Lismont C, Walton P. (2017). The Peroxisome-Mitochondria Connection: How and Why?. Int J Mol Sci. 2017;18(6):1126. Published 2017 May 24. doi:10.3390/ijms18061126
(10) Snehal N. Chaudhari & Edward T. Kipreos, (2017). Increased mitochondrial fusion allows the survival of older animals in diverse C. elegans longevity pathways. Nature Communications vole 8, Article number: 182
(11) Wilhelmi de Toledo F, Grundler F, Sirtori CR, Ruscica M., (2020) Unravelling the health effects of fasting: a long road from obesity treatment to healthy life span increase and improved cognition. Ann Med. 2020 Aug;52(5):147-161. doi: 10.1080/07853890.2020.1770849. Epub 2020 Jun 10. PMID: 32519900.
About this Article
Fasting and a longer lifespan, A. Whittall
©2018 Fit-and-Well.com, 11.Jan.2019. Updated. 10.Dec.2020. https://www.fit-and-well.com/wellness/fasting-and-a-longer-lifespan.html
Tags: lifespan, longevity, longer life, fasting, alternate fasting, restricted calorie diet, health benefits, diabetes type 2, weight loss, anti-inflammatory effects, oxidative stress, mitochondria, peroxisomes.
This Webpage
Subject: Fit-and-Well.com. Alternate fasting could extend your lifespan: Following an intermittent fasting diet or a calorie restricted-diet can extend lifespan by up to 27% in mice. Exercising has a similar effect. The key to a longer lifespan in rodents and nematodes is exercise and fasting. These impact the mitochondria causing them to remain fused or elongated, improving ATP production efficiency and protecting mitochondria from age-related decay. ROS (free radicals) are also kept under control.






